Metal soaps
Formation of metal soaps in paint layers exemplify a very complex chemical process, which results from the interaction of fatty components of the binder with certain metal elements in pigments (typically Pb or Zn). The resulting soaps can crystallize, aggregate and protrude to the surface of the painting, where they cause cracking of the paint layer and its falling off. In other cases, the soaps are responsible for colour fading caused by increased transparency of the affected paint layers. Although saponification affects mainly oil paintings, it can occur also in fatty tempera. However, the progression of soap formation in individual cases depends on numerous factors involving the type of reactive pigments, composition of the binder, character of admixtures, concentration of individual components, relative humidity, temperature, type of support, previous cleaning and conservation treatments, etc.
The long-term experimental research focuses on clarification of particular factors affecting saponification in various paint systems. Besides that, analytical methods suitable for detection of secondary changes in paint layers are tested. For proper identification of degradation products, it is often necessary to synthesize and characterize reference compounds due to the paucity of reliable structural data.
Recent papers:
Kočí E., Rohlíček J., Kobera L., Plocek J. Švarcová S., Bezdička P.: Mixed lead carboxylates relevant to soap formation in oil and tempera paintings: the study of the crystal structure by complementary XRPD and ssNMR. Dalton Transactions 48 (2019), 12531-12540.
Švarcová S., Kočí E., Bezdička P., Garrappa S., Kobera L., Plocek J., Brus J., Šťastný M., Hradil D.: Uncovering the Lead Formate Crystallization in Oil-Based Paintings. Dalton Transactions 49 (2020), 5044 – 5054.
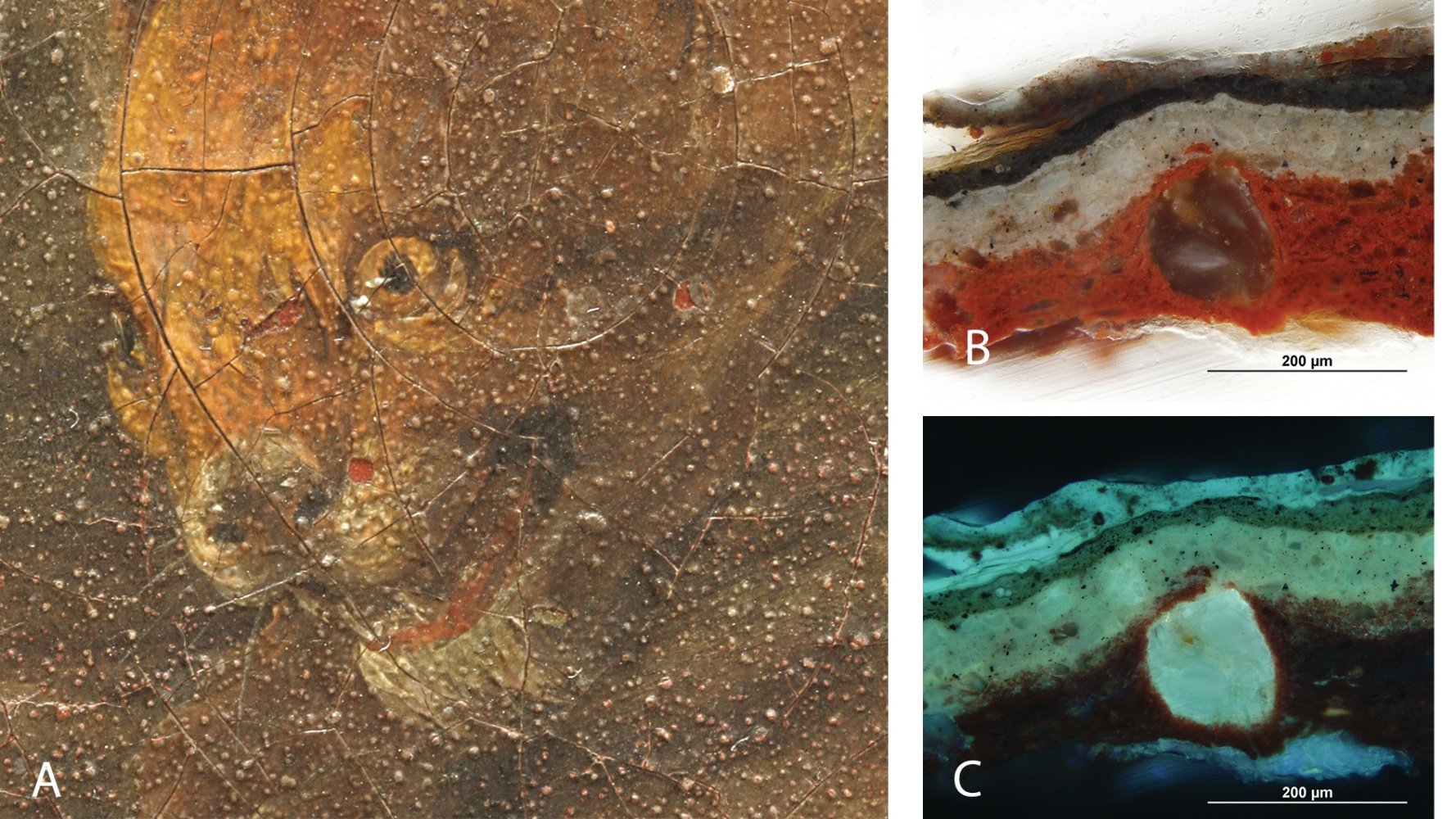
Blisters on the surface of the painting (A) as a result of metal soaps’ formation in the red ground layer (by the interaction of oil with minium), growing and protruding to the surface, as shown on microphotos in visible (B) and UV light (C)


